Experimental Petrology’s Long Road to Respect
|
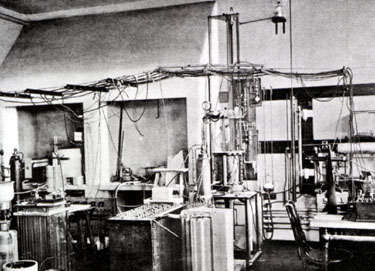
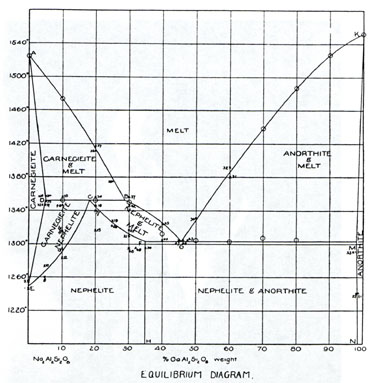
Experimental petrology owes much to the foundation of the Geophysical Laboratory in 1905, and later to the groundbreaking work of Norman L. Bowen in his classic 1928 book, The Evolution of the Igneous Rocks. While a respected area of geoscience research today, before the 1940's, according to science historian Carl-Henry Geschwind, the Laboratory and Bowen’s work were largely ignored or unappreciated. The Laboratory was established at a time when top igneous petrologists wanted to gain “exact science” prestige for their field, like chemistry or physics. With the leadership and support of first director Arthur L. Day, the Geophysical Laboratory chose a more experimental basis for research over field study. The first experiments focused on discovering which phases minerals or liquids are stable in a given chemical system while paying close attention to temperature and composition. Starting in 1909, the data for temperature-composition phase diagrams were attained by the quench method. To do so, a small amount of powdered material of a known composition was heated to a known temperature and held there until the material reached chemical equilibrium. The sample was next quenched, which means it was quickly cooled to room temperature. This process freezes the higher-temperature phase relations so that they can be looked at and identified under a microscope. |
|||
|
|
|||
|
|||
|
|
|||
|
This reductionist approach to understanding the behavior of high-temperature silicate melts provided exactness to the Geophysical Laboratory’s work and aided the concerns of industries like cement, ceramics, and glass. A large step in improving the quality of the Laboratory’s work arrived in 1912 with Norman L. Bowen. His first studies were comparable to the work of others on phase equilibria in the Laboratory, but within a year, he began to produce experimental results on untouched materials and theories. His first papers pertained to his work with Olaf Anderson on clino-enstatite and the theoretical effects of forsterite resorption on magmatic differentiation. Bowen set out in a new direction in 1914. He dedicated his work to proving that “the settling out from basic magma of certain minerals is the dominant control in the differentiation of most igneous rocks.” To accomplish this lofty goal, he used mainly physical experiments. According to Bowen, experimentation and a thorough understanding of reaction relationships was key to comprehending magmatic differentiation. His most famous work, The Evolution of the Igneous Rocks, describes the many different routes magmas might take during differentiation by explaining experimental results from the Geophysical Laboratory. |
|||
|
|
|||
|
|||
|
|
|||
|
To defend himself on this subject, Bowen stated that “volatiles are of little importance in the problem of igneous differentiation." He believed that less than one percent of natural magmas were volatiles. This amount was so small that phase relations determined from volatile-free systems would not be considerably affected. Despite these allegations, most petrologists remained skeptical. As a whole, American petrologists disregarded the Geophysical Laboratory’s phase diagrams and Bowen’s theories and writings. By the early 1940s, the Geophysical Laboratory’s petrology program had yet to find widespread acceptance. This was all to change in the next two decades. Making the petrology program more accessible to the rest of the scientific community was one of the first items on director Day's successor's to-do list. Unlike Day, L. H. Adams sought input from both the Laboratory’s staff and outside petrologists. Adams also persuaded staff members to undertake geological field expeditions as well as laboratory investigation. These developments in communication made the Laboratory more receptive to field petrologists’ needs. Another change Adams put into effect was the object of study for Geophysical Laboratory petrologists. He stressed the importance of analyzing the under pressure conditions of equilibrium between crystals and liquids in the presence of volatile components as well as nonvolatile. Adams also encouraged experimentation on natural rocks. |
|||
|
|
|||
|
|||
|
|
|||
|
References:
Further Reading:
|